Open here for our page navigation
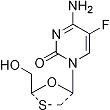
Emtricitabine
4-Amino-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-(2R-cis)-2(1H)-pyrimidinone
CAS Number [143491-57-0]
Appearance off-white crystalline powder
Molecular Formula C8H10FN3O3S
Molecular Weight 247.25
Assay 99%
Specific Rotation [α] 25/D -129o
Loss on Drying < 1.0%
Heavy Metals < 20 ppm
Impurities < 1.0%
Burning Residue < 0.1%
Emtricitabine was discovered by Dr. Dennis Liotta, Dr. Raymond Schinazi and Dr. Woo-Baeg Choi of Emory University and licensed to Triangle Pharmaceuticals by Emory in 1996. Triangle Pharmaceuticals was acquired in 2003 by Gilead Sciences, who completed development and now market the product with the brand name Emtriva®.
It was approved by the FDA July 2, 2003. It is very similar to 3TC and cross-resistance between the two is near-universal.
Emtricitabine (FTC), with trade name Emtriva® (formerly Coviracil), is a nucleoside reverse transcriptase inhibitor (NRTI) for the treatment of HIV infection in adults.
Emtricitabine is also marketed in a fixed-dose combination with tenofovir (Viread®) under the brand name Truvada®. A fixed-dose triple combination of emtricitabine, tenofovir and efavarenz (Sustiva®, marketed by Bristol-Myers Squibb) is in development.